pH is a measure of how acidic or basic a solution is. It stands for “potential of hydrogen” and is defined as pH = -log10[H+]. The pH scale runs from 0 to 14 (7 is neutral); each whole-number change in pH corresponds to a tenfold change in [H⁺]. (Lower pH means more acidic.) This concept is important in chemistry and daily life.
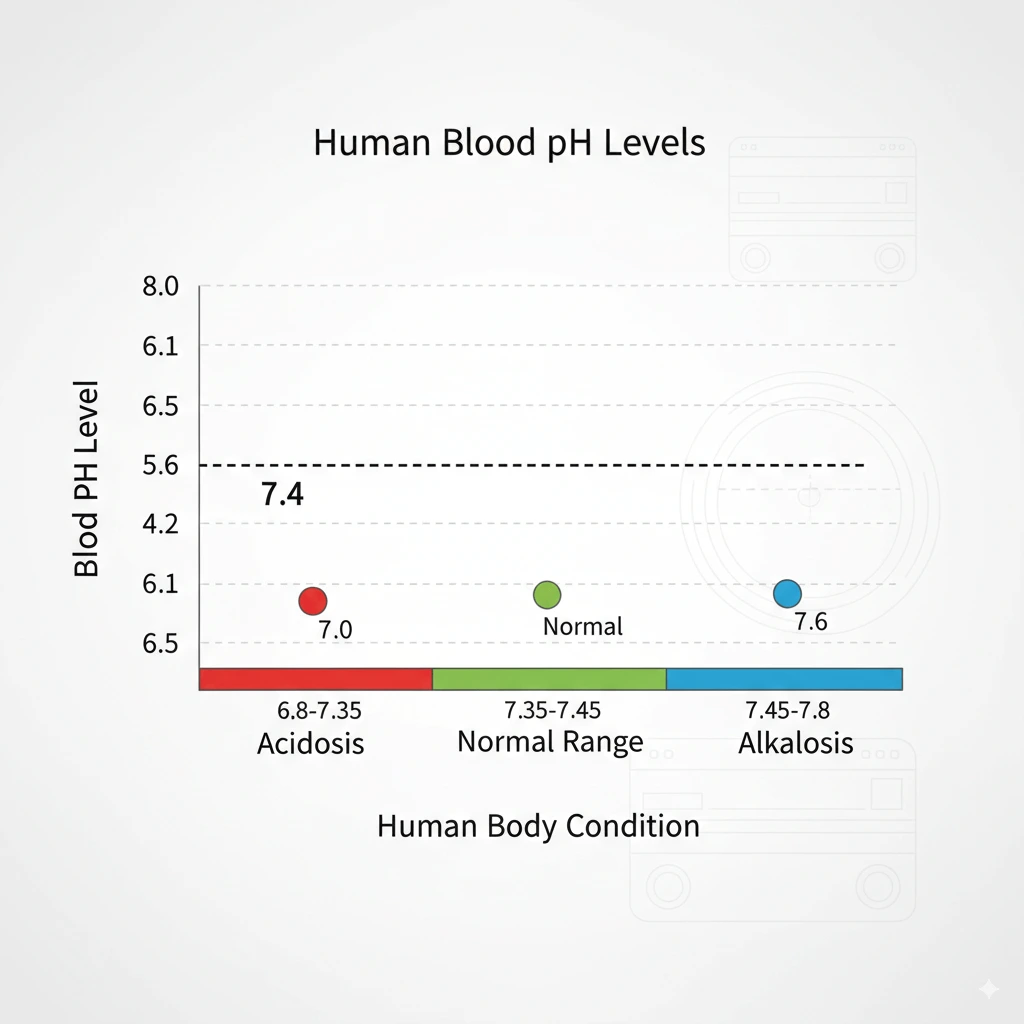
For example: Soil pH affects plant growth, and blood pH is tightly regulated around 7.4 for health.
Molarity: Concentration and pH
Molarity (M) is the number of moles of solute per liter of solution Molarity (M) is the number of moles of solute per liter of solution, as explained in our molarity vs molality guide. In acid/base chemistry, knowing the molarity of an acid or base tells us the starting concentrations of H⁺ or OH⁻. For a strong (fully dissociated) acid, [H⁺] equals the acid’s molarity. For a strong base, [OH⁻] equals the base’s molarity. We then use these ion concentrations to find pH.
Calculating pH of Strong Acids and Bases
Strong acids and bases dissociate completely in water.
- For strong acid (like HCl):
[H⁺] = acid molarity.
- Example: 0.010 M HCl → [H⁺] = 0.010 M, pH = –log (0.010) = 2.00.
For a strong base (like NaOH):
[OH⁻] = base molarity. Find pOH = –log [OH⁻], then pH = 14 – pOH.
- Example: 0.050 M NaOH → [OH⁻] = 0.050 M, pOH = –log (0.050) ≈ 1.30
- pH = 14 – 1.30 = 12.70.
These straightforward calculations assume full dissociation, but in more complex cases (like titration), you may need to apply methods from our molarity in titration guide. (In very dilute solutions, water’s own [H⁺] (~10-7 M) can slightly affect pH, but most problems ignore this effect.)
Practical Applications of pH
Biology & Medicine
Enzymes and bodily processes need specific pH ranges. Human blood is maintained around pH 7.4; small deviations (acidosis/alkalosis) are dangerous. Stomach acid (pH ~1–2) is strong to aid digestion.
Environment
Soil pH affects nutrient availability and plant health. Water pH influences aquatic life (very low or high pH can harm fish and microbes). Acid rain (pH ~ 4) can damage ecosystems, whereas unpolluted rain is around pH 5.0–5.5.
Food & Industry
pH controls fermentation, taste, and preservation. In dairy, measuring pH helps detect spoilage or infection; cheese and yogurt require specific acidity. Baking relies on pH (e.g. baking soda needs acid to produce CO₂). Food canning guidelines use pH to prevent bacterial growth (foods with pH >4.6 need special processing).
Tips for Accurate pH Calculations
- Significant figures: In a pH value, only the decimal places count for sig figs. For example, [H⁺] = 2.20×10⁻³ M (3 sig figs) gives pH = 2.657, usually rounded to 2.657 (three decimal places).
- Use base-10 logs: Always use log₁₀, not ln. Double-check your calculator mode when taking logarithms.
- Handle dilutions properly: If a solution is diluted or mixed, use our dilution calculator to quickly recalculate the new molarity (M₁V₁=M₂V₂) before finding pH.
Conclusion
Calculating pH from molarity means connecting concentration to acidity. For strong acids/bases, it’s straightforward: take –log([H⁺]) or 14-log([OH⁻]). We’ve highlighted practical contexts (biology, environment, food) where pH matters. By practicing these steps and using tools like the calculator of molarity or the calculator of normality, students can confidently tackle pH problems.

